Abstract
Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is a curative option for patients with AML in first complete remission (CR1), but is limited by the procedure-related toxicity and mortality. Thus, one could be reluctant for proceeding to Allo-HSCT in elderly patients, especially in the intermediate ELN risk group. While indications of Allo-HSCT for AML are consensual for younger patients, the benefit in older patients remains a matter of debate. In this analysis of a 10-year single center transplantation program for AML, we aim to show the feasibility and study the benefit of Allo-HSCT for CR1 AML in patients over 60 years of age.
Inclusion criteria were: patients between 60 and 70 years of age; AML in first complete remission after intensive chemotherapy; ELN intermediate or unfavorable. Allo-HSCT was evaluated as a time dependent variable in survival calculations and in a multivariate Cox model adjusted on age (continuous), ELN group (intermediate vs. unfavorable) and time interval between induction therapy and CR1 (continuous). In addition, we used a multistate model as follow: initial state for all patients was "No Allo - CR" with time 0 at the time of CR after induction therapy. From initial state, patients can transit to "Allo - CR" at the time of transplantation, or go through 2 absorbing states: the non-relapse death in absence of Allo-HSCT (No Allo - NRM) and leukemia relapse in absence of Allo-HSCT (No Allo - Relapse). Similarly, once transplanted (i.e. in the state "Allo - CR"), patients can move to "Allo - Relapse" or "Allo - NRM" if they present relapse or non-relapse death, respectively. The model allows the dynamic prediction of probability for a patient to be in a specific state considering specific initial state and time.
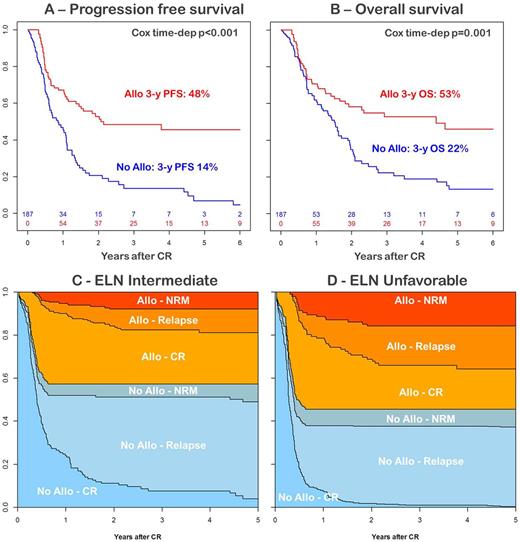
We analyzed all 187 consecutive patients who matched inclusion criteria. Median age was 65 years (range: 60-70). ELN risk was intermediate and unfavorable in 139 and 48 patients, respectively. While all had theoretical indication for Allo-HSCT in first CR, 83 were actually transplanted (44%). Reasons to not proceed to Allo-HSCT were patient or physician decision, absence of donor, relapse or NRM. In the entire cohort, 3-year progression free survival (PFS) was 48% after Allo-HSCT vs. 14% without Allo-HSCT, and 3-year overall was 53% after Allo-HSCT and 22% without Allo-HSCT. The time dependent Cox model showed that these differences are statistically significant in favor of Allo-HSCT (PFS: HR [95%CI]: 0.45 [0.29-0.69] p<0.001, Figure 1A; OS: HR [95%CI]: 0.49 [0.32-0.76] p=0.001, Figure 1B). Using the same Cox model, cumulative incidence of relapse (CIR) was strongly reduced after Allo-HSCT (HR [95%CI]: 0.26 [0.16-0.45] p<0.001), while Allo-HSCT was associated with a trend for higher NRM (HR [95%CI]: 2.19 [0.90-5.37] p=0.085). The favorable impact of Allo-HSCT was similar in both intermediate and unfavorable ELN risk groups, in terms of disease control (CIR-intermediate: HR [95%CI]: 0.27 [0.15-0.50] p<0.001; CIR-unfavorable: HR [95%CI]: 0.26 [0.08-0.86] p=0.028) and survival (OS-intermediate: HR [95%CI]: 0.55 [0.33-0.90] p=0.018; OS-unfavorable: HR [95%CI]: 0.36 [0.15-0.90] p=0.028). Multistate model showed that 3 years after CR, few patients were still alive in CR without Allo-HSCT (i.e. in the initial "No Allo - CR" state), whatever the ELN risk group (intermediate: 8% Figure 1C; unfavorable: 1% Figure 1D). In addition, the multi state model showed that, considering a landmark at 4 months after CR, patients who received Allo-HSCT had lower probability of relapse at 3 years (24% and 35% in intermediate and unfavorable groups, respectively) compared to those who did not (49% and 54% in intermediate and unfavorable groups, respectively).
We conclude that Allo-HSCT for patients >= 60 years of age with CR1 AML is routinely feasible (44% actually transplanted), and significantly improves outcome in both intermediate and unfavorable ELN risk groups. In contrast with the setting of younger patients, long term survival is rare (less than 10%) without Allo-HSCT, even in intermediate risk group, supporting that Allo-HSCT remains the first curative option for these patients.
Charbonnier: Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal